Background: Natural killer (NK) cells are innate lymphocytes that mount immune responses against viral infection and malignant cells. Circulating NK-cells can be generally divided into two groups, CD56 bright and CD56 dim, with the latter constituting ~90% of NK-cells in peripheral blood. While CD56 bright NK-cells are considered less mature and capable of producing more cytokines, CD56 dim population represents more differentiated cells with greater cytotoxicity. Transcription factor (TF) regulatory circuits were proposed for CD56 bright and CD56 dim NK-cells, indicating that TCF1/LEF1 and BACH2 are important in the former population and that Blimp1 and MAF are master TFs in the latter group. Blimp1 (encoded by PRDM1) has been previously found to regulate terminal differentiation of B-cells and CD8 + T-cells. In NK-cells, deletion of PRDM1 has been shown to promote cell growth and survival in vitro, and its loss of function was commonly detected in NK-cell lymphomas. Here, we employed a multi-omics approach to investigate the role of PRDM1 in the differentiation of human primary NK-cells.
Methods: We used CRISPR-Cas9 system to knock out PRDM1 in primary human NK-cells isolated from PBMCs of healthy donors and performed whole transcriptome sequencing. ChIP-seq was employed to investigate the global binding spectrum of PRDM1 in primary human NK-cells cultured with K562-mbIL21-Cl9 feeder cells or with IL-2 alone. We also performed integrative genomics analyses to examine the transcriptomic regulation of NK-cell differentiation by PRDM1.
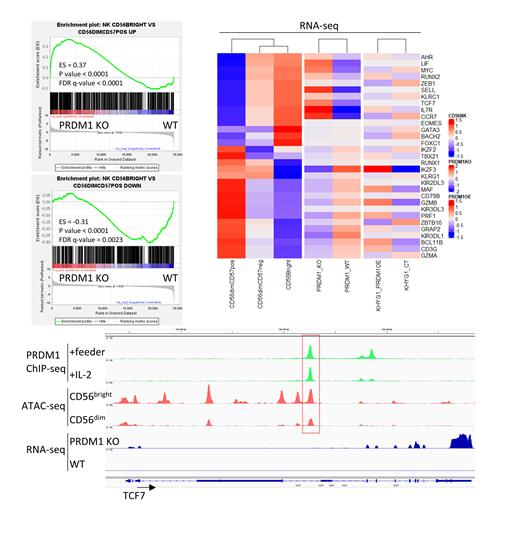
Results: Gene set enrichment analyses (GSEA) demonstrated that PRDM1 KO resulted in enrichment of genes highly expressed in CD56 bright cells and depletion of genes upregulated in CD56 dim NK-cells. Specifically, we found that PRDM1 deficient NK-cells expressed CD56 bright NK-cell master TFs at higher levels, such as TCF7, RUNX2, and MYC. In contrast, TFs that play an important role in more differentiated CD56 dim NK cells were downregulated in PRDM1-KO cells, including IKZF3, MAF, and TBX21. Notably, BCL11B, a TF that has been reported to promote canonical and adaptive NK-cell differentiation, was significantly downregulated along with its target gene ZBTB16 when PRDM1 was deleted. Moreover, genes encoding cytotoxic molecules such as PRF1 and GZMB were also repressed in PRDM1-KO NK-cells. Together, these results indicate that PRDM1-KO NK-cells closely resembled the less mature CD56 bright NK-cells than the CD56 dim terminally differentiated counterpart.
ChIP-seq analysis of PRDM1 revealed that many of the differentially expressed genes (DEGs) between PRDM1 KO and WT cells were directly bound by PRDM1. When integrated with publicly available ATAC-seq datasets, we found that some of these PRDM1 bound sites showed differential chromatin accessibility between CD56 bright and CD56 dim NK-cells. To explore the transcriptional program regulated by PRDM1, we utilized a regulated system to re-express PRDM1 in KHYG1, an NK-cell lymphoma cell line that is sensitive to PRDM1 overexpression. RNA-seq analysis showed that the DEGs between KHYG1 with short-term re-expression of PRDM1 and control cells overlapped with DEGs between PRDM1 KO vs WT NK-cells. These genes included BCL11B, MAF, PRF1, and GZMB. Therefore, PRDM1 directly regulates genes that are crucial in NK-cell differentiation.
Furthermore, we detected upregulation of memory T-cell or progenitor exhausted T-cell signature genes in PRDM1 KO NK-cells compared with WT, including TCF7, SELL, CCR7, and IL7R. Consistent with this, GSEA analysis demonstrated enrichment of TCF1 + progenitor exhausted T cell or memory T cell upregulated genes in PRDM1 deleted NK-cells, suggesting that PRDM1 KO NK-cells may share some features with memory T-cells or progenitor exhausted T cells. Importantly, MYB, which was found to be essential in progenitor exhausted T cells, was also a direct target of PRDM1 and was upregulated in PRDM1 KO NK-cells. GSEA also showed that PRDM1 depletion in NK-cells led to upregulation of genes highly expressed in mast cell, Th1 cell, B cell, or CD8 + T cell compared to NK cells, which may indicate a role of PRDM1 in maintaining lineage commitment.
Conclusions: Our findings collectively show that PRDM1 may be a master TF that regulates human NK-cell differentiation and functions through direct transcriptional regulation of key target genes.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal